


Joseph R. Anticaglia MD
Medical Advisory Board
The COVID-19 Sweepstakes has become the most famous international, scientific race in history. From China to the United States, from Canada to Australia, scientists are off and running to be the first to get a COVID-19 vaccine to the market. Forget the roses. The prize money in the COVID’s winner circle is worth billions of dollars to the pharmaceutical company that wins this race.
But the grand prize is ending the coronavirus-19 pandemic and this humanitarian trophy is priceless. Thus far, COVID-19 virus (SARS CoV-2) has outsmarted the body’s immune system and its so-called “Department of Defense.” It has sickened and murdered people around the globe. The statistics change daily, but in late July there were 17,406,644 million COVID-19 cases in 188 countries, with 675,213 deaths, and the United States accounted for approximately one fifth of that total. Neither time zone, time of year nor continent is off limits to this elusive coronavirus.
Some pharmaceutical companies have taken an early lead in the COVID Sweepstakes by commencing phase three clinical trials. Others are in phase one, but it’s still a wide open race. The leaders have yet to round the first turn in this international race. The drug companies will bunch-up over the next several months before they get to the finish line.
What might we look for: Will the vaccine be in the form of an injection? Will it be necessary to receive two or more injections? Is any company developing a nasal spray vaccine for COVID-19? If it does provoke immunity with antibodies against COVID-19, how long will it last? How safe and effective will it be? Is there a danger of getting the disease from the vaccination? Can we afford the price of the vaccine? When will it become available?
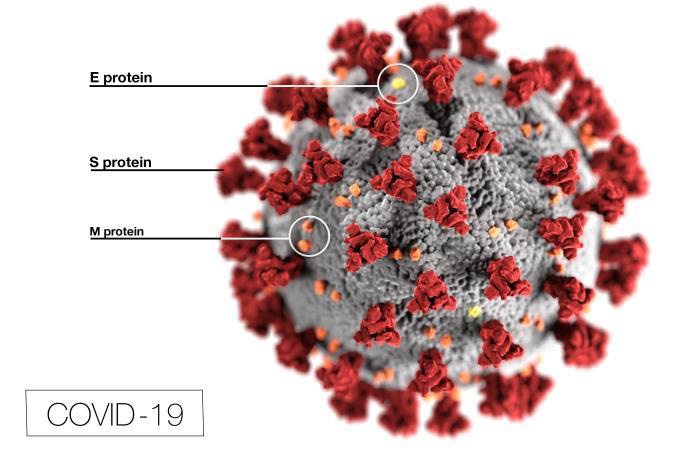
Researchers are familiar with the coronavirus family of viruses such as, the common cold, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERV). They’re working on ways to disrupt the attachment of the coronavirus’ spike-like S-proteins to the surface of the human, ACE2 receptors cells. A vaccine that disables the S-protein would prevent it from attaching itself to human cells, prevent it from entering the cells and prevent it from replicating (reproducing).
Before a vaccine is made available to the public, it goes through clinical trials to assure its safety and effectiveness. Different research approaches are utilized to develop and produce the vaccine. Thereafter, in the U. S., the vaccine needs to receive approval from the Federal Drug Administration before it can be marketed to the public.
Global pharmaceutical companies are partnering to increase the likelihood of being the first to deliver a safe and effective vaccine to the public. What follows are some of the methods used in an attempt to solve the COVID-19 puzzle. It’s to be noted, at present, there is NO VACCINE that’s available to prevent this disease.
This is an attempt to produce a non-virulent, a killed version of the virus that causes COVID-19. These whole-cell vaccines produce a weaker immune response when compared to live vaccines. They’re not capable of replicating (reproducing) within the cell. It often requires multiple doses and booster shots to achieve long-term immunity. There’s no risk of acquiring the disease. Inactivated vaccines have provided protection against influenza (shot only), hepatitis A, polio (shot only) and rabies.
Drug companies are working on an attenuated (weakened form) of the live COVID-19 virus to induce a satisfactory immune response. These live, whole-cell vaccines are capable of replicating within a cell and require extensive testing.
There’s a risk of transmitting the virus to the non-immunized individuals. Of concern is its use in people with a compromise immune system. Live, attenuated vaccines given by injection are usually effective after one dose. They protect people against mumps, measles and rubella (MMR), chickenpox, smallpox and yellow fever.
Researchers are working on gene-based vaccines for human diseases. They’re manipulating and instructing fragments of the coronavirus’ DNA or RNA genetic code to produce S-proteins. These proteins provoke the production of viral antibodies that can be useful against COVID-19. The studies are in the experimental stage and aim to develop a DNA vaccine or a RNA vaccine. Currently, no such vaccine has been approved for human use. No virus is introduced into the body.
Subunit vaccines use specific pieces of the microorganism to elicit an immune response. These vaccines may also use the “product” of microorganisms, for instance, their toxins to provoke the immune response. In addition, scientists may combine the specific pieces of the organism with sugars or yeast to enhance the immune response.
They’re safer than attenuated vaccines. The vaccines can be used on persons with compromised immune systems and those with chronic health problems. People may require booster shots to maintain protection against such diseases.
HHS noted these vaccines are used to protect against:
Toxoid vaccines use the harmful product, namely, the toxin made by the microorganism that causes the disease. In this instance, the immune response is targeted to the toxin instead of the whole microorganism. You need booster shots to maintain ongoing protection against Tetanus and Diphtheria.
The COVID-19 competition is fierce. In July of this year, the U. S. ordered the Chinese consulate in Houston, Texas to be closed after it accused Chinese personnel of coronavirus research espionage. Technology has significantly shortened the timeline from phase 1 of clinical trials to the time the vaccine will be available to the general public.
Optimistically, precautions are in place to preserve the safety and effectiveness of the vaccine when the winner crosses the finish line in this crucial international sweepstakes. The grand prize: ending the COVID-19 pandemic!
ACE2 — (angiotensin-converting enzyme2 — ACE2 receptors).
This article is intended solely as a learning experience. Please consult your physician for diagnostic and treatment options.